“This technique reduced the difficulties to less than half”
Prof. Chiapasco has used many surgical techniques to gain new bone height and width. Recently, he published his clinical experience with 3D titanium mesh. What is key to success for him?
Prof. Chiapasco, what convinced you to try Yxoss CBR®?
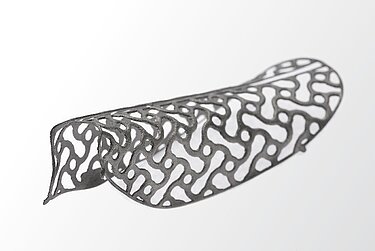
The available titanium meshes can be difficult to manipulate and fixate. I hoped that Yxoss CBR® would simplify the surgical procedure.
Did it?
Yes. And I have also significantly reduced operating times. Moreover the stability and accuracy of the reconstruction are amazing. You can visualize the reconstruction in 3-D before you start surgery and calculate the amount of graft material needed. And by using Yxoss CBR® I avoid more traumatic harvesting and sampling of bone blocks. I only use bone chips – mainly harvested from intraoral sites – and Geistlich Bio-Oss®.
How much height and width can you gain with Yxoss CBR®?
Theoretically, there are no significant limits to bone augmentation, neither vertically nor horizontally. In some cases, I gained 10 mm in height. But we can arbitrarily divide bone augmentations into three categories: 1–3 mm, 4–6 mm, and > 6 mm. The bigger, the more difficult. Vertical augmentations can be more demanding than horizontal, especially for less experienced surgeons.
How would you convince a colleague to try Yxoss CBR®?
While it is important to be an expert in GBR, this technique reduces the difficulties to less than half and is predictable, effective, and precise. Try it to believe it.
In major bone augmentation, dehiscences are a frequent problem. How often do you experience such complications when using Yxoss CBR®?
If we included all exposures, the incidence is approximately 20%. But most of them do not compromise the outcome, especially if they occur in later stages of graft integration. Severe early dehiscences with loss of the grafts happened in less than 5% of our cases.
What is key to avoid dehiscences?
Many factors can reduce this risk:1) Working in sterile conditions; 2) Accurate and significant flap release to get a tension-free and hermetic suture; 3) Good quality of soft tissues; 4) No load with removable prostheses on the regenerated areas.
If dehiscences occur, how do you proceed? Will they disturb bone healing?
If the dehiscence occurs immediately after surgery, the risk of relevant loss of the grafting material is higher. The particulated graft is more exposed and is not yet integrated. I do not recommend resuturing the flaps, though. It could increase the infection risk as the mesh might be covered by a film of bacteria. If signs of infection are evident, in particular suppuration, I recommend removing the mesh, allowing the grafting material to cover with granulation tissue and re-evaluating after a few weeks. Despite this unfavorable event, part of the original graft may still integrate.
If the dehiscence occurs later, there is a high chance that granulation tissue has formed below the exposed parts and will protect the graft. In this case, oral hygiene reasures such as chlorhexidine mouth rinses and frequent recalls will allow to maintain the majority of the graft. Antibiotics are helpful in the first week after surgery. But in the early phases the graft is poorly vascularized, and antibiotics hardly reach the target.
Mesh removal can be difficult. What is your experience with Yxoss CBR®?
Mesh removal is one of the most delicate phases of the whole regeneration procedure. During healing, the bone graft can even “submerge the mesh” with new bone formation, thus rendering its removal more complex. Plus, soft tissues overlying the mesh are generally thin and penetrate through the mesh. Extreme accuracy must be ensured to separate soft tissue and the consolidated bone grafts from the mesh.
Do you have advice on how to make mesh removal easier?
Wait at least seven to eight months and…good and trained hands, and delicate, attentive measures!!!
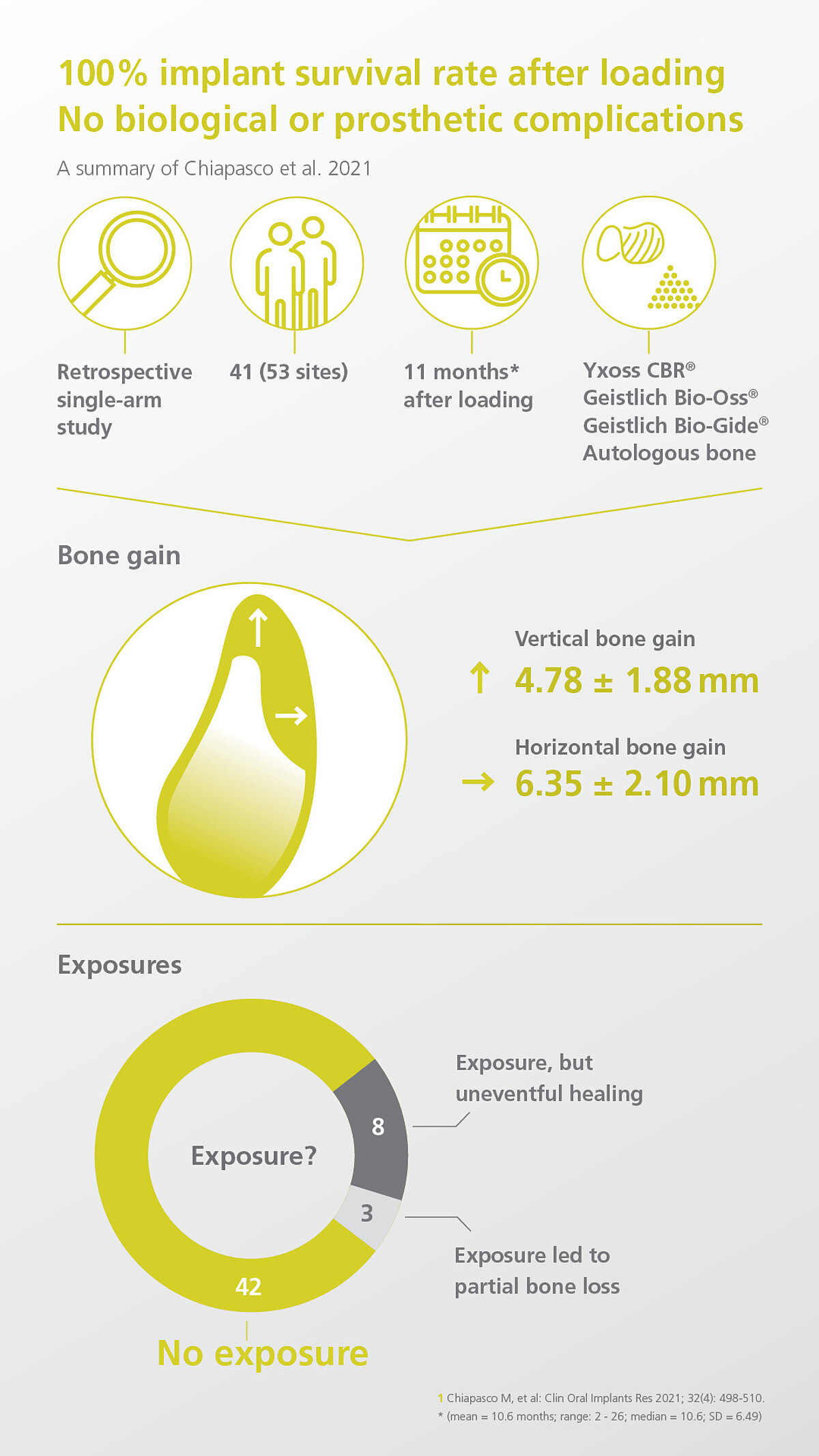
- Chiapasco M, et al: Clin Oral Implants Res 2021; 32(4): 498-510. (clinical study)