3 Key protocols for horizontal and vertical bone defects
Dental implant therapy has shown tremendous long-term bone and soft-tissue stability when sufficient bone volume is available at the time of implant insertion. On the other hand, inadequate bone volume around implants can be a significant risk factor.
Several techniques for augmenting bone defects have been developed. They include protocols both for bone augmentation before implant placement (two-stage approach) and simultaneously with implant placement (one-stage approach), along with adjunctive materials such as bone blocks, particulate bone substitutes, autologous bone chips, form-stable devices, collagen and dense polytetrafluoroethylene (d-PTFE) membranes, fixation screws and pins, and bone harvesting devices.
Guided Bone Regeneration: Backed by Science
Among the techniques for horizontal and vertical bone augmentation, GBR is one of the most investigated and evidence-based approaches. It produces predictable results and high, long-term implant survival rates.1,2 Studies show that the survival rates of implants placed into augmented bone do not differ from the survival rate of implants placed into pristine bone.3 The technique is based on sound biological principles with a step-by-step clinical protocol. The surgical complication rate ranges from low to high, categorizing the procedure at times as technique sensitive. The rate of complication largely depends on proper patient selection and diagnosis, detail-oriented surgical steps, careful patient follow-up and optimal choice of biomaterials.
For smaller bone defects a simultaneous GBR approach delivers the same results as a staged GBR approach. With large horizontal ridge atrophy and vertical defects, a staged protocol seems to be more predictable and produce better results.4
GBR can be conducted with a 1:1 mixture of autologous particulate bone and anorganic bovine bone substitute (Geistlich Bio-Oss®) in combination with a native collagen membrane (Geistlich Bio-Gide®) or, for larger horizontal or vertical defects, in combination with a form-stable titanium-reinforced d-PTFE membrane.
The following sections describe the three main protocols used in our clinical practice and our training programs.
GBR Protocol 1: Implant dehiscence and fenestration defects
In 1992, we published our first study on the GBR protocol around dehisced implant surfaces in 12 patients,5 and one of the patients who was treated for amelogenesis imperfecta is still in follow-up after 29 years with stable crestal bone and successful implants.
In 2018, we published modifications and the results of 45 consecutive cases (63 implants) treated with our layered bone graft and GBR protocol, and followed the patients for 30 months after loading. No patient dropped out of this study, stable bone and soft tissue was noted and no implant or prosthesis failed (see details in Box 1).6
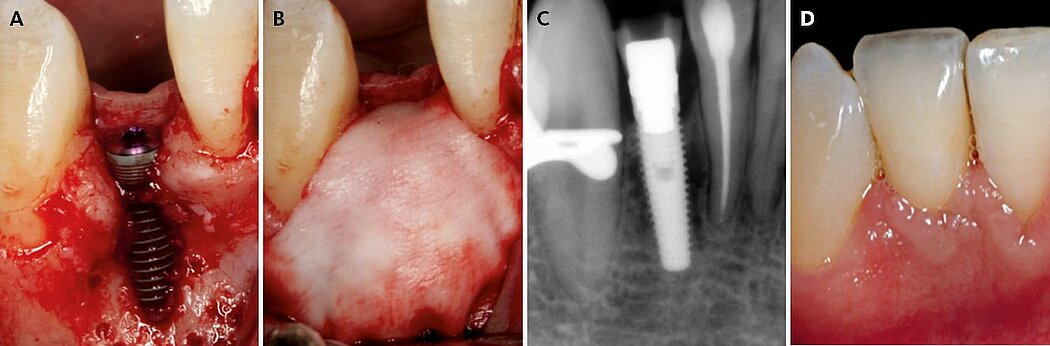
The key point is to apply this GBR protocol to smaller bone defects, and an accurate CBCT diagnosis is critical for selecting a bone volume between 4 to 6 mm in width. The combination of autologous bone in contact with the implant and anorganic bovine bone on top is the key to success (Fig. 1). While the autologous bone has osteoinductive and osteogenic properties, the anorganic bovine bone maintains the volume and contour in the long-term. Collagen membranes are advantageous compared to expanded polytetrafluoroethylene (e-PTFE) membranes in this indication because of the favorable soft-tissue healing and because they do not have to be removed. Their lack of form stability can be overcome by the bone mixture and accurate fixation of the membrane that allows immobilization of the graft material.
Figure 1. Clinical case pictures of a patient treated in 2007 with a simultaneous resorbable GBR treatment protocol 1 in a thin healed ridge of 4 mm width showing stable crestal bone and soft tissue margin after 12 years of function.7
GBR Protocol 2: Larger horizontal defects
In 1995, we published the use of space-making titanium-reinforced e-PTFE membranes for large horizontal defects and this was later modified to resorbable membranes in one-wall large horizontal defects.8 For these larger horizontal defects, a staged GBR procedure is safer and more predictable than a simultaneous GBR and implant approach. The bone graft (now a larger volume mixture of autologous bone and anorganic bovine bone) can be covered with a native collagen membrane (Geistlich Bio-Gide®) or with a titanium-reinforced d-PTFE membrane depending on the severity of the bone deficiency. In general, one-wall large buccal defects with a CBCT bone width of 3 to 4 mm can be grafted and covered with a collagen membrane fixed with pins both lingually/palatally and buccally. In cases with severe two-wall horizontal resorption and with a CBCT bone width of less than 3 mm the autograft/bone substitute mixture is covered with a non-resorbable d-PTFE membrane fixed with screws on the periphery.
Healing periods of six to eight months are used in this GBR protocol, and over 5 mm of new horizontal bone is created, as evidenced in multiple clinical studies.9,10 This creates enough bone width for predictable implant placement. Soft-tissue management before, during and after the GBR technique is essential to ensure healthy, thick soft tissue for tension-free flap closure and to create enough keratinized tissue for implant success.
GBR Protocol 3: Vertical bone defects
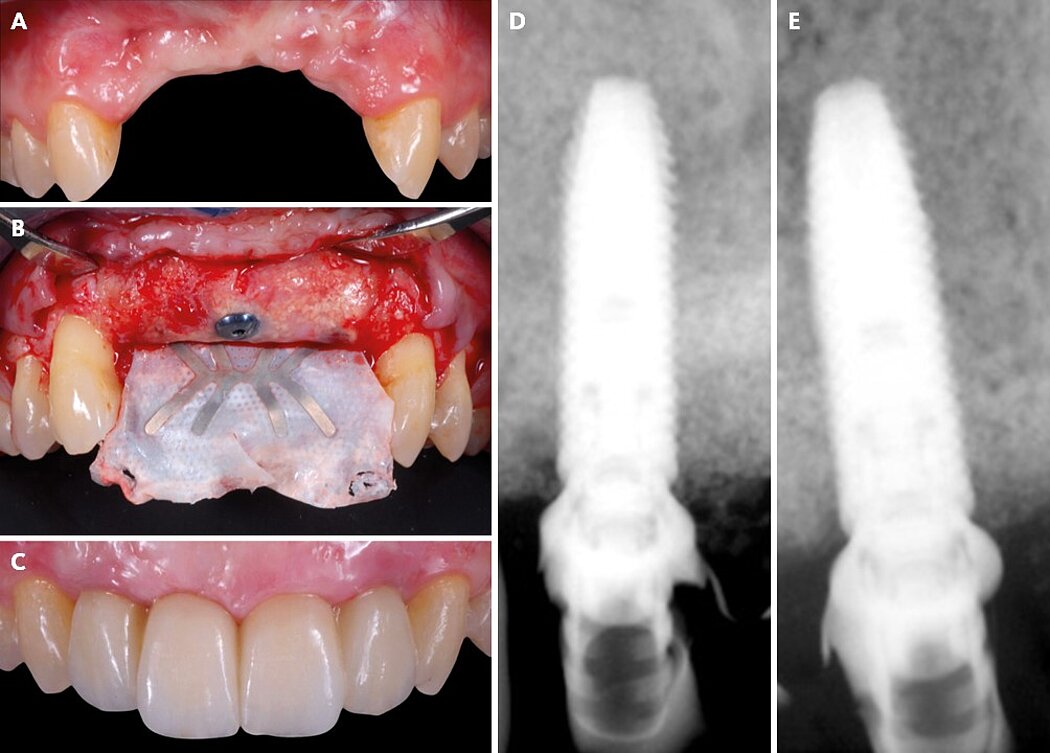
Vertical ridge augmentation is the most challenging of the GBR protocols, as it aims to regenerate large amounts of vertical and horizontal bone with little or no bone walls to use as a base for the bone formation. For the blood supply to reach the full distance from native bone into the outer part of the grafted area and for complete mineralization to take effect, a longer healing period of 9 to 12 months is needed. In addition, to protect the bone graft from soft-tissue invasion during the healing period, a space making device with long-term cell exclusion and a thick and advanced soft tissue flap is needed to provide a closed healing environment for the GBR-grafted area (Fig. 2).
Figure 2. Clinical case pictures of a patient treated with a non-resorbable d-PTFE staged GBR protocol 3 treatment for a severely vertical resorbed ridge in the anterior maxilla.
When treating vertical bone defects, a titanium-reinforced e-PTFE membrane was designed, and we tested the first prototype designs in 1993, which were published for the first time in 1995 in our animal and human studies.8-11 The large amount of clinically documented cases has shown that a maximum vertical gain of 12 mm is possible with a mean of more than 5 mm and a horizontal gain of 8 to 10 mm, which is sufficient in most cases to place an implant in the optimal esthetic position (see details in
Box 2).12,13
Key to success
Placing an implant into vertically augmented bone is rather challenging, because the bone is still early in its mineralization nine months after augmentation. Therefore, implant placement in this indication should be done by an experienced surgeon, and implant selection has to be performed carefully.
As in all GBR procedures, it is mandatory to select the right kind of patient for this challenging procedure. Periodontal problems have to be resolved before the treatment starts, and compliance with recall intervals has to be guaranteed with minimal to no plaque deposits. Regarding bone graft biomaterial, we always stick with the proven combination of autologous bone plus xenogeneic bone substitute – for the combination of osteoinductive properties and long-lasting volume stability – plus collagen or PTFE membranes depending on the defect (horizontal/vertical/combined). The need for and benefit of adding platelet-rich plasma (PRP) or platelet-rich fibrin (PRF) is still to be fully seen but has possible early wound healing benefits that could help with flap closure (see details in Box 3).






References
- Lutz R, et al.: Clin Oral Implants Res 2015; 26 Suppl 11:103-22.
- Sanz-Sanchez I, et al.: J Dent Res 2015; 94:128S-142S.
- Donos N, et al.: J Clin Periodontol 2008; 35:173-202.
- Meloni SM, et al.: Clin Implant Dent Rel Res 2017; 19:38-45.
- Jovanovic SA, et al.: Int J Oral Maxillofac Implants 1992; 7(2):233-45.
- Meloni SM, et al.: Eur J Oral Implantol 2018; 11(1): 89-95.
- Magne P, et al.: J Prosthet Dent 2008; 99(1):2-7.
- Jovanovic SA, Nevins M: Int J Periodontics Restorative Dent 1995; 15(1):56-69.
- Urban IA, et al.: Int J Periodontics Restorative Dent 2013; 33(3):299-307.
- Urban IA, et al.: Int J Oral Maxillofac Implants 2011; 26(2):404-14.
- Jovanovic SA, et al.: Int J Oral Maxillofac Implants 1995; 10(1):23-31.
- Urban IA, et al.: Int J Oral Maxillofac Implants 2014; 29:185-93.
- Simion M, et al.: Clin Oral Implants Res 2001; 12(1):35-45.