Less chair time, better esthetics: matrix vs. autologous graft
In a randomized clinical trial, Dr. McGuire et al. have compared the long-term outcomes of recession coverage with Geistlich Mucograft and free gingival graft. What was the result?
Palatal grafts are considered the gold standard for soft tissue augmentation. Why did you conduct a clinical trial1 with a head-to-head comparison between Geistlich Mucograft® and the so-called gold standard?
The point of using free gingival graft (FGG) is stability over time. That’s the key. So, of course we wanted to compare to the gold standard.2 It turned out that Geistlich Mucograft® not only prevented recession just as well (ΔRec = −0.07 ± 1.26 mm for Geistlich Mucograft® and −0.17 ± 0.78 mm for FGG, p = 0.710), the results were maintained in the long-term (6+ years), while also generating keratinized tissue width (KTw). And over time there was no difference in the ΔKTw between therapies.
We have patients who had free gingival grafts in the mid-60s, and those grafts are still working, so this long-term comparison was important.
Of course, we may never find a harvest graft substitute as absolutely perfect as live tissue, but in today’s world, and especially weighing patient desires and outcomes, almost as good is fine, especially because it is quicker and easier to do – and patients prefer it.
Your study showed Geistlich Mucograft® and free gingival graft are equally successful in increasing the width of keratinized tissue. What is the minimum width for a healthy soft tissue verdict? What are the consequences when this minimum width is not available?
I remember Jan Wennström made the statement that nobody ever lost a tooth because of a lack of keratinized tissue width. That made me wonder, “So what am I doing?”
But keratinized tissue width has its place. Tougher tissue helps patients with maintenance. Recently, the American Academy of Periodontology redefined its statement about keratinized tissue width: “... a minimum amount of keratinized tissue is not needed to prevent attachment loss when optimal plaque control is present.”3 However, if plaque control is suboptimal, a minimum of 2 mm of keratinized tissue width is needed.”4 Pini Prato’s long-term studies are fantastic, and 2 mm is often cited as the amount needed.5,6
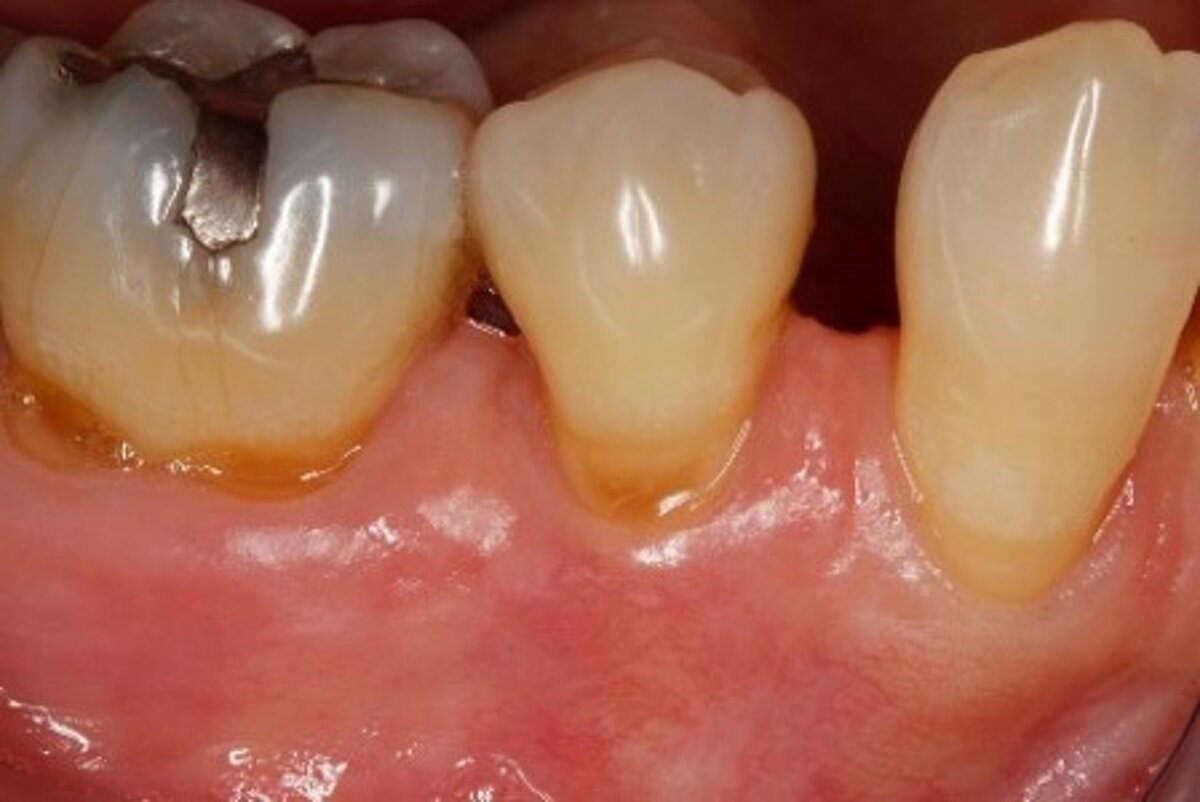
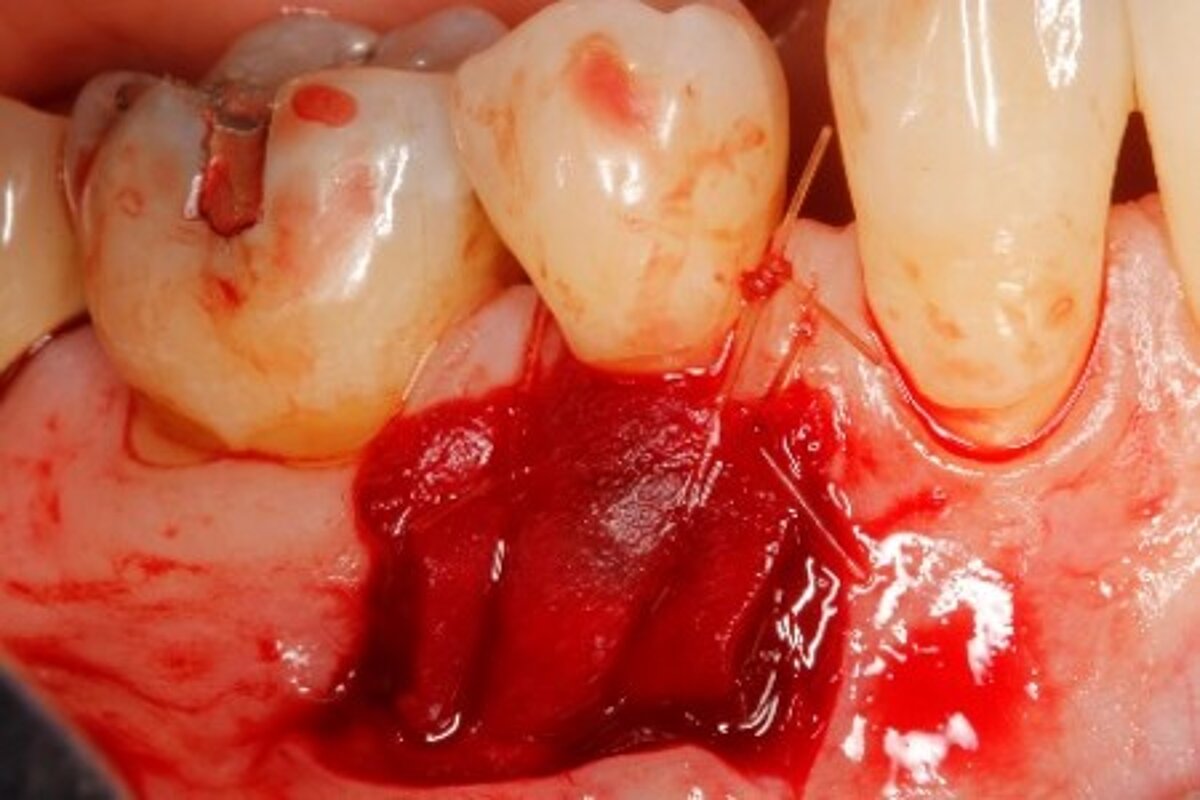
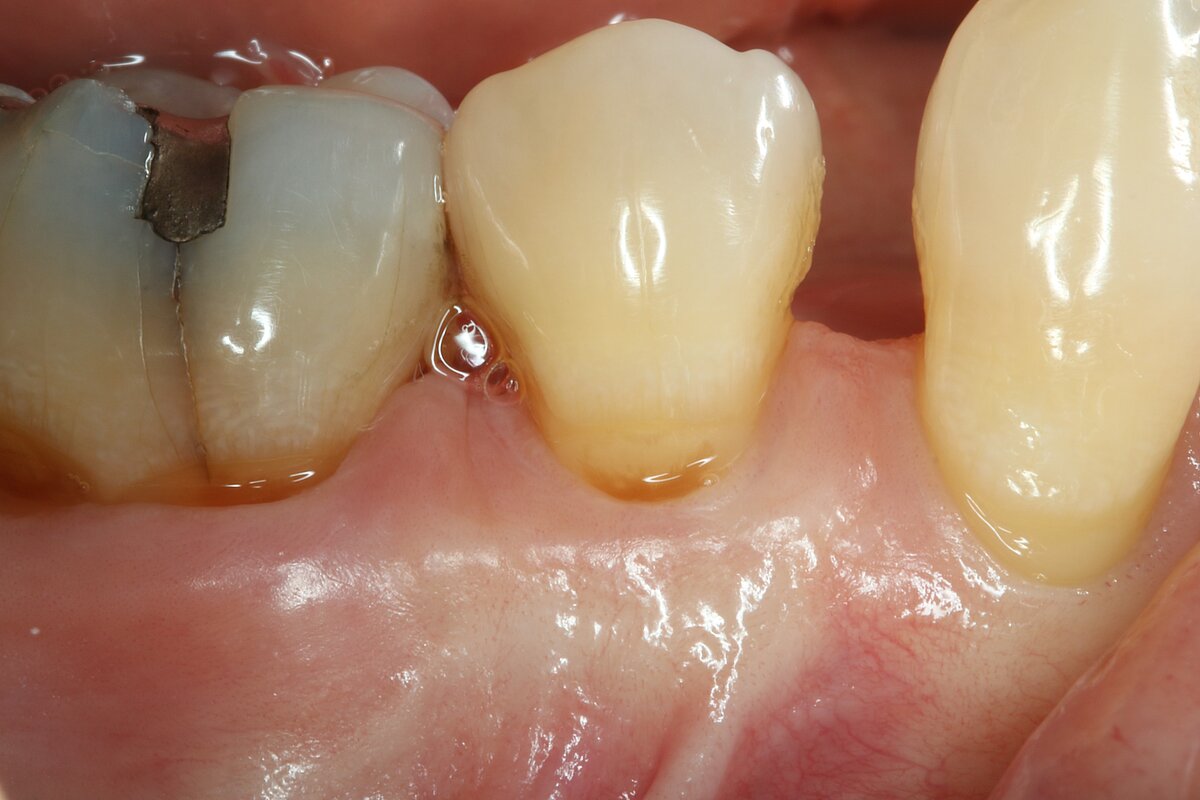
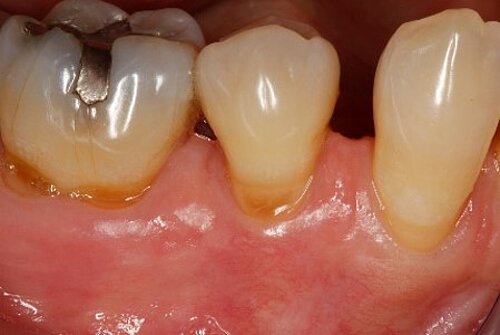
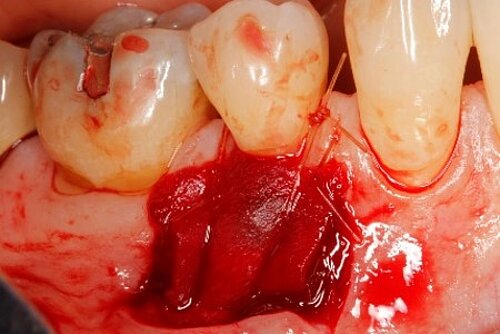
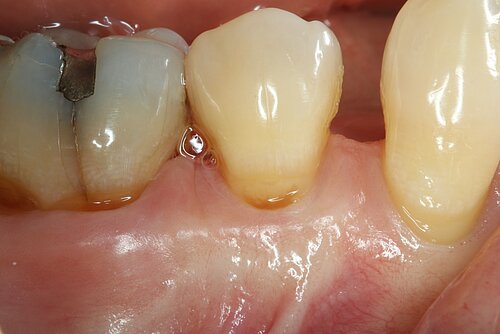
You showed the color and texture of the sites treated with Geistlich Mucograft® were better matched with surrounding tissue in the long-term. Why is this so?
When we take tissue from the palate, it retains its phenotype. Some dentists have been trained to expect to see this “tire patch”, but that’s not the way gingival augmentation procedures are headed in the future. With Geistlich Mucograft® you get the tissue that belongs there - site appropriate gingival phenotype.
Was the chair-time different?
It (Geistlich Mucograft®) does take less time! It may be hard to quantify, as in clinical studies, taking photos and measurements usually complicate the procedure. But compared with FGG, Geistlich Mucograft® is definitely faster, and with an unlimited supply.1
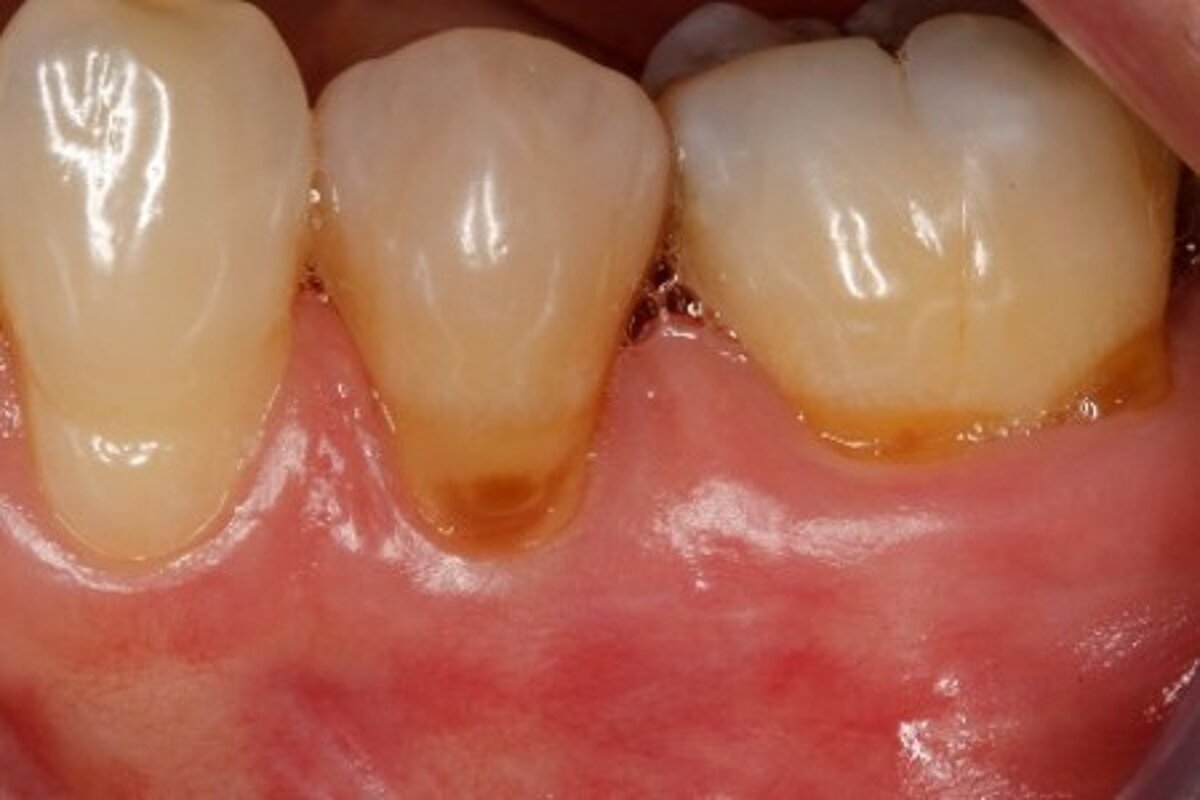
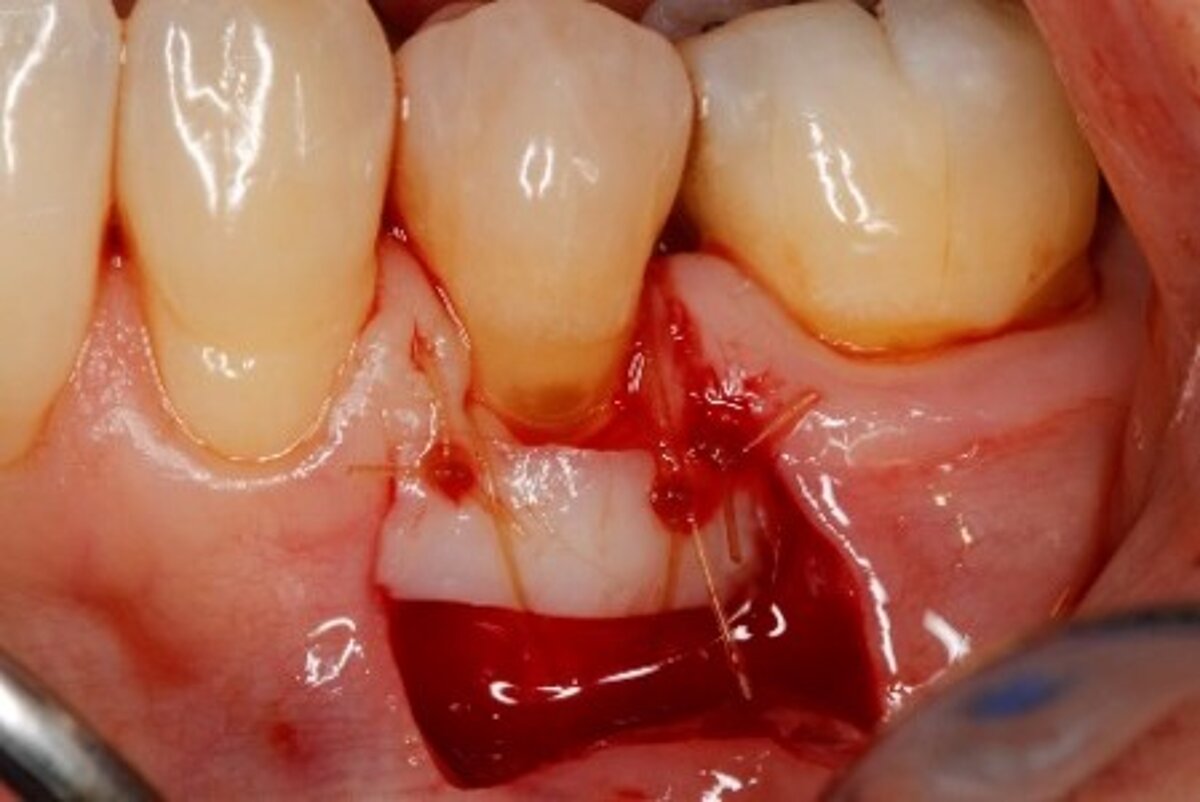
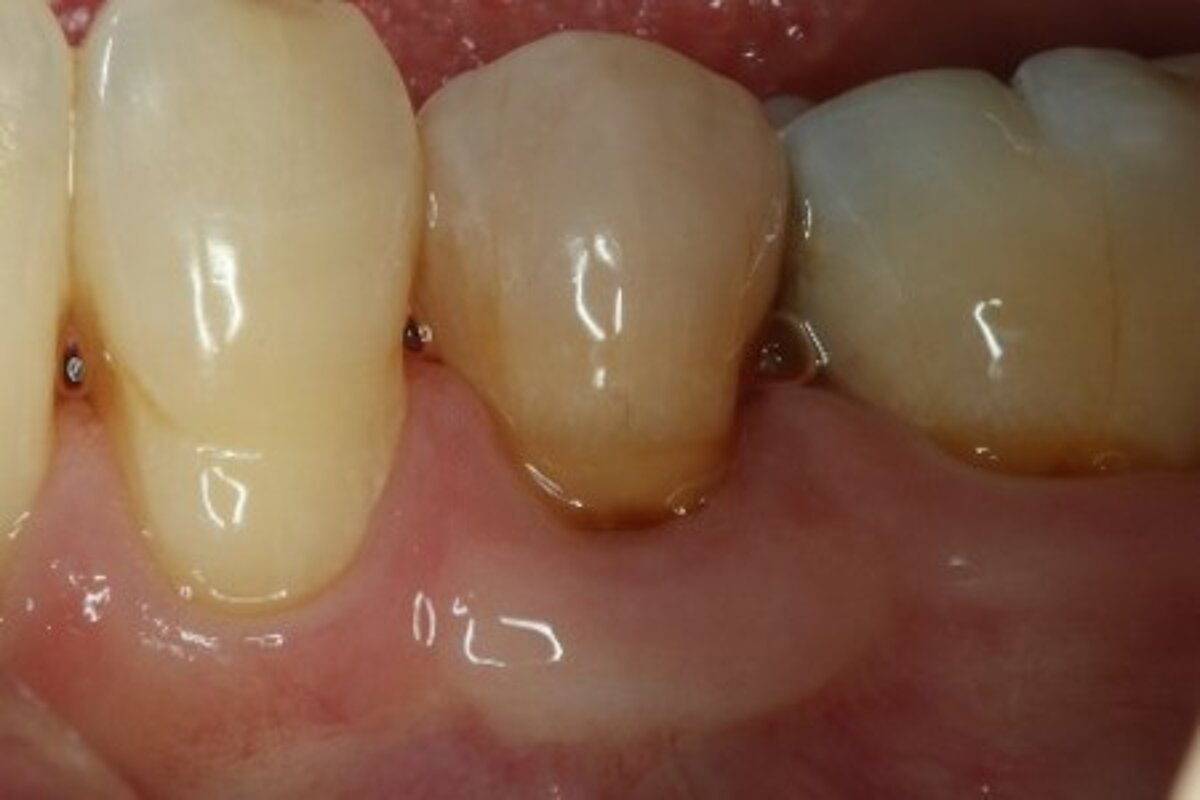
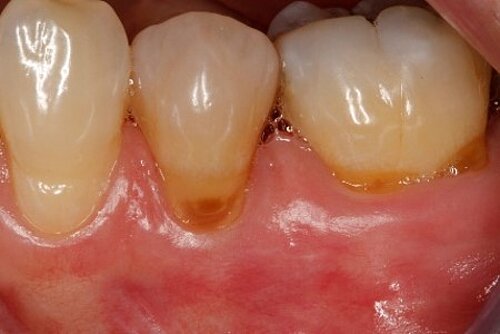
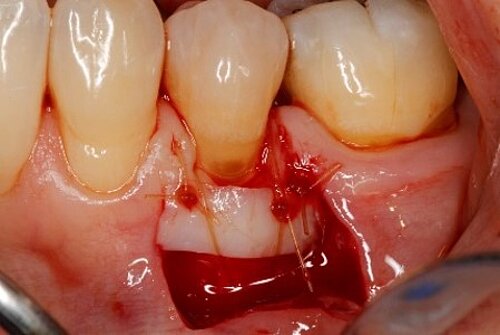
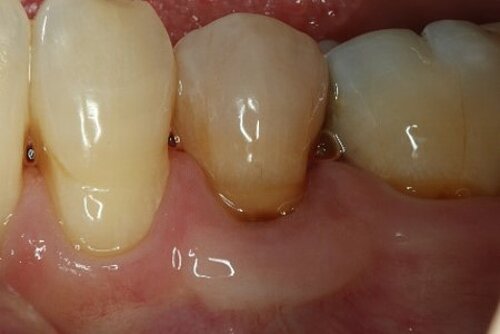
Did procedure time impact patient satisfaction? What other factors influenced patients’ opinions?
Sure, but there are also the three Cs: comfort, cosmetics and convenience. There is less pain, results look better,7and I don’t have to stage surgery to be able to harvest enough FGG, which is not just chair-time but the number of times patients are in the chair.
References
- McGuire MK, et al.: 2020 Dec 21. Epub ahead of print. (clinical study)
- Thoma DS, et al.: Clin Oral Implants Res. 2009;20 Suppl 4:146-65. (systematic review)
- Kim DM and Neiva R: J Periodontol. 2015;86(S2):S56–S72. (systematic review
- Scheyer ET, et al.: J Periodontol. 2015;86(2 Suppl):S73-S6. (consensus report)
- Pini Prato GP, et al.: J Periodontol. 2018;89(3):265-74. (clinical study)
- Agudio G, et al.: J Periodontol. 2017;88(7):634-42. (clinical study)
- McGuire MK, et al. : J Periodontol. 2014;85:1313-9. (clinical study)